Please select your Region.
Please select your Region.
Jul 08, 2019
Most urine testing consists of qualitative tests utilizing urine test strips for the measurement of proteinuria, urinary sugar and occult blood as well as sediment testing for the purpose of analyzing test samples which have been judged to possess abnormalities during qualitative testing. Urine sediment tests are performed visually by laboratory technicians using a microscope to examine, categorize and measure formed elements inside urine samples (red blood cells, white blood cells, other cells and bacteria). These tests are considered important criteria in the diagnosis of patients with kidney and urinary tract diseases. Contrariwise, microscope testing involves complicated tasks including sample centrifugation and preparation. Additionally, a large portion of the testing relies on the skills of the lab technician. Therefore, issues remain in standardizing the determination of results. Given this background, the need for an automated sediment analyzer has been increasing year by year.
ARKRAY, Inc.'s (hereunder, ARKRAY) "AUTION EYE AI-4510" urine sediment analyzer uses a flow-type image measurement method* to capture images of formed elements inside urine samples, then automatically extracts, analyzes, categorizes and measures those formed element images, thereby providing urine sediment test data. The captured images can be checked via the operation screen, allowing these images to be utilized as reference information as well as an alternative to microscope testing. This allows for a reduction in the number of required microscope tests, permitting lab technicians to focus on test samples subject to microscope tests. Furthermore, a collection of representative formed elements called the "atlas image library" comes installed as a standard, allowing for standardization of formed element determination and for in-facility study as an educational tool. The "AUTION EYE AI-4510" has a compact design, allowing for installation even in labs with limited space, and its operation screen which displays test results has been designed with operability in mind.
Since successfully designing the world's first automated test strip-based urine analysis system in 1972, ARKRAY has continued to provide the market with automated urinalysis systems, urine test strips and test data management systems for over 40 years. Adding the "AUTION EYE AI-4510" to ARKRAY's product portfolio will allow ARKRAY to provide total support for a full set of procedures, from urine dispensing and qualitative testing to sediment testing and quantitative testing. (Refer to page 3 for related products). ARKRAY will continue to streamline laboratory work flows and contribute to the improved efficiency of testing operations in order to expand its presence in the urine testing market and grow its business.

○ Fully automated measurement utilizing a "flow-type image measurement method"
· Fully automatic measurement is possible; the operator is only required to set the sample and press the start button
· Formed elements are extracted from captured elements and automatically categorized using a unique algorithm
· The only consumable regularly required is sheath solution; extremely convenient and cost-efficient
· No infectious solid waste such as cartridges are produced, thus no disposal fees are incurred
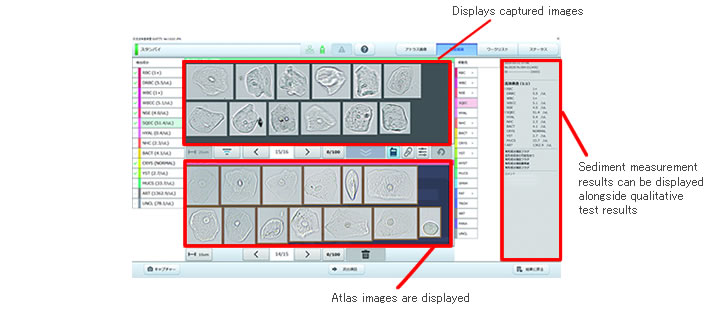
○Integrates various support functions on the operation screen
· The confirmation screen, which displays measurement results, utilizes an easy-to-view layout with intuitively designed operability
· The summary screen, which displays multiple elements on one screen, allows the operator to easily grasp the frequency of occurrence of each element
· It is also possible to display the sediment testing results of a sample alongside its qualitative test results
○Convenient "atlas image library" inside
· A collection of representative formed elements called the "atlas image library" comes installed, allowing for side-by-side comparisons with captured images on the same screen
· Images unique to each user can be added, allowing the atlas image library to be used as a tool both for the standardization of element determination and as an in-facility learning tool
○Support for quality control
· Specialized controls are available allowing for quality control
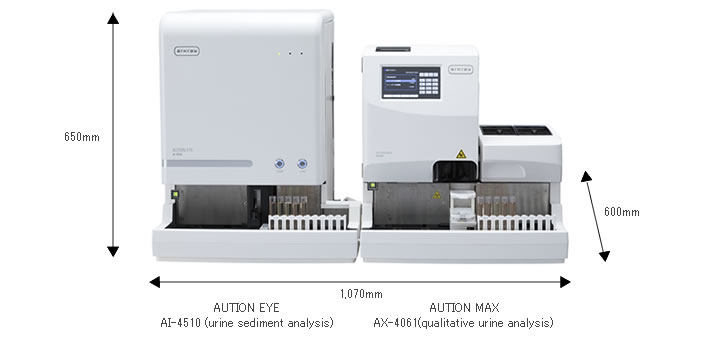
○Compact footprint allows for simple transportation and setup
· Utilizes a sample transportation system, allowing for connection with the qualitative urine analyzer "AUTION MAX AX-4061"
· A compact footprint of approximately 1 meter in width is still possible even with both products installed

* Images of urine samples transported to the flow cell via sheath solution are captured, and from those images only the formed elements are automatically extracted. Using a unique categorization algorithm, the extracted images are analyzed, categorized, measured and presented as sediment test data.
| Name | Urine sediment analyzer "AUTION EYE AI-4510" | |
|---|---|---|
| Release date | July 8, 2019 (Monday) | |
| Specifications | ||
| Measurement target | Urine | |
| Measurement item | Red blood cells, white blood cells, white blood cell mass, squamous cells, non-squamous cells, hyaline casts, other casts, bacteria, yeasts, mucous threads, sperm, crystals | |
| Required sample size | 2 mL or more | |
| External dimensions | W: 530 x D: 600 x H: 650 mm | |
| Weight | 60 kg (including sampler unit) | |
| Sale price | Recommended delivery price: 13,500,000 yen (before tax) | |
| Registration number | 25B1X00001000058 | |
| Category | Class I (general medical devices)/Specific Maintenance Medical Device | |
![]()